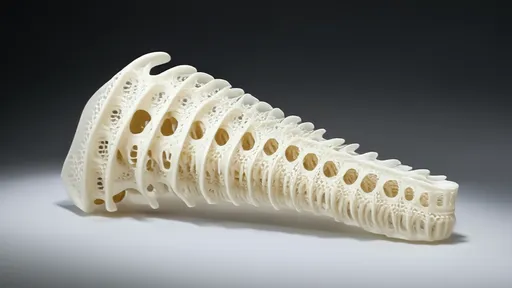
The field of biomedical engineering has witnessed a groundbreaking advancement with the development of 3D-printed gradient porous scaffolds inspired by natural bone structures. These innovative implants, designed to mimic the complex architecture of human bones, are poised to revolutionize orthopedic and dental treatments. By leveraging the precision of additive manufacturing, researchers have created scaffolds with meticulously controlled porosity gradients that mirror the mechanical and biological properties of native bone tissue.
Nature's blueprint serves as the foundation for these remarkable synthetic structures. Human bones exhibit a sophisticated gradient in porosity, transitioning from dense cortical bone on the exterior to spongy trabecular bone in the interior. This natural design provides an optimal balance of strength and nutrient transport capabilities. Scientists have spent years attempting to replicate this intricate architecture, and now with advanced 3D printing technologies, they've achieved unprecedented success in creating biomimetic structures that closely resemble the body's own tissues.
The manufacturing process involves sophisticated computer modeling to design scaffolds with precisely engineered pore sizes that gradually change across the structure. This gradient approach addresses one of the major challenges in bone regeneration - creating implants that simultaneously provide mechanical support while allowing for vascularization and tissue ingrowth. The outer layers with smaller pores offer the necessary structural integrity, while the inner regions with larger pores facilitate cell migration and nutrient diffusion.
Material science plays a crucial role in the development of these scaffolds. Researchers are experimenting with various biocompatible polymers, ceramics, and composite materials that can be precisely deposited by 3D printers. The choice of material significantly affects the scaffold's degradation rate, mechanical properties, and biological performance. Some teams are incorporating bioactive components like hydroxyapatite, a natural mineral found in bones, to enhance the scaffold's osteoconductive properties and improve integration with host tissue.
Clinical applications for these gradient porous scaffolds are vast and transformative. They show particular promise in treating large bone defects resulting from trauma, tumors, or congenital disorders where natural healing is insufficient. The ability to customize each implant's size, shape, and porosity to match individual patient anatomy represents a significant leap forward in personalized medicine. Dental applications include jawbone reconstruction and periodontal treatments, where the gradient structure can support both the mechanical demands of chewing and the biological requirements of tissue regeneration.
The biological performance of these scaffolds has demonstrated remarkable results in preclinical studies. The gradient porosity appears to guide cellular behavior in ways that uniform scaffolds cannot, directing stem cell differentiation and promoting organized tissue formation. This spatial control over tissue regeneration mimics natural bone development processes, potentially leading to faster healing and better functional outcomes. Researchers have observed improved vascularization in gradient scaffolds compared to traditional designs, addressing one of the critical limitations in large bone defect repair.
Challenges remain in optimizing these scaffolds for widespread clinical use. The relationship between pore geometry, mechanical properties, and biological response requires further investigation to establish design rules for specific applications. Long-term studies are needed to understand how the scaffolds degrade over time and how the newly formed bone remodels in response to physiological loads. Researchers are also working on incorporating growth factors or other biological cues into the scaffolds to further enhance their regenerative capabilities.
The integration of advanced imaging technologies with 3D printing has opened new possibilities for patient-specific implants. CT or MRI scans can be used to create digital models of bone defects, which are then translated into customized scaffold designs. This approach ensures optimal fit and function while maintaining the critical porosity gradients needed for successful regeneration. Some research groups are exploring the combination of scaffolds with patient-derived cells to create living implants that could potentially accelerate the healing process even further.
Industrial and academic collaborations are accelerating the translation of this technology from the laboratory to the clinic. Several companies have begun developing commercial products based on gradient porosity principles, while regulatory agencies work to establish appropriate standards for these novel medical devices. The economic potential is significant, as the global market for bone graft substitutes continues to grow alongside an aging population and increasing prevalence of bone-related disorders.
Looking ahead, researchers envision even more sophisticated designs that incorporate multiple gradients - not just in porosity but also in stiffness, bioactive molecule distribution, and cellular composition. The ultimate goal is to create smart scaffolds that can dynamically interact with the body's healing processes, adjusting their properties in response to the local biological environment. Such advances could blur the line between synthetic implants and natural tissue, leading to truly integrative solutions for bone regeneration.
The development of 3D-printed gradient porous scaffolds represents a convergence of materials science, biology, and engineering that promises to transform regenerative medicine. As the technology matures, it may not only improve outcomes for bone repair but also inspire similar approaches for regenerating other complex tissues. The biomimetic principles underlying these scaffolds highlight the value of looking to nature for solutions to some of medicine's most persistent challenges.
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025