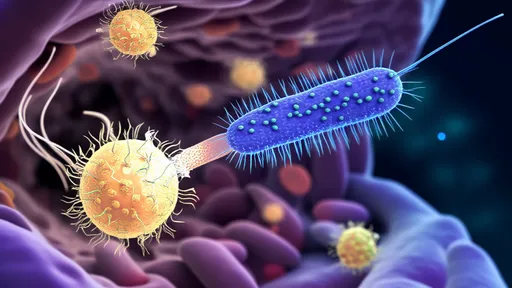
In the ever-evolving landscape of medical science, a groundbreaking approach is emerging as a potential game-changer in the fight against antibiotic-resistant bacteria: bacteriophage therapy. Often dubbed "virus missiles" for their precision targeting capabilities, these microscopic predators are being harnessed to selectively eliminate harmful gut pathogens while preserving beneficial microbiota. This revolutionary treatment paradigm could rewrite the rules of infectious disease management.
The human gut houses trillions of microorganisms collectively known as the microbiome, maintaining a delicate balance crucial for health. When pathogenic bacteria like Clostridioides difficile or antibiotic-resistant E. coli disrupt this equilibrium, traditional antibiotics often act like indiscriminate bombs, wiping out both harmful and beneficial bacteria. Bacteriophages, nature's ancient viral predators, offer an elegant alternative by targeting specific bacterial strains with surgical precision.
What makes phage therapy particularly exciting is its evolutionary origins. These viruses have been hunting bacteria for billions of years, constantly adapting alongside their prey. Scientists are now learning to harness this natural arms race for therapeutic purposes. Unlike broad-spectrum antibiotics that drive resistance across multiple species, phages typically attack only their specific bacterial targets, minimizing collateral damage to the microbiome.
The therapeutic process begins with isolating and characterizing the problematic bacterial strain from a patient's gut microbiome. Researchers then screen vast phage libraries or environmental samples to identify viruses capable of infecting and lysing the target pathogen. These selected phages are purified and sometimes genetically optimized before being administered orally or via colonoscopy, where they multiply exponentially as they destroy their bacterial hosts.
Recent clinical trials have demonstrated remarkable success stories. In one notable case, a patient suffering from a persistent E. coli infection that resisted all antibiotics achieved complete recovery after a tailored phage cocktail treatment. Another study involving recurrent C. difficile infections showed phage therapy could break the cycle of reinfection by specifically targeting toxin-producing strains while sparing other gut microbes.
The potential applications extend beyond treating active infections. Prophylactic use of phages could protect vulnerable populations like chemotherapy patients or those undergoing organ transplants from opportunistic gut pathogens. Researchers are also exploring engineered phages that can deliver targeted payloads - from CRISPR components that edit bacterial genomes to enzymes that break down antibiotic resistance genes.
Despite these promising developments, significant challenges remain. Regulatory frameworks struggle to categorize these living therapeutics that evolve during treatment. Manufacturing standards need development to ensure consistent phage preparations. There's also the scientific hurdle of bacterial resistance to phages, though combination therapies and rapid phage adaptation may overcome this.
The gut microbiome's complexity presents another layer of difficulty. With hundreds of bacterial species interacting in dynamic ecosystems, predicting how introduced phages will behave requires sophisticated modeling. Some researchers advocate for "phage banks" containing characterized viruses against common pathogens, while others pursue synthetic biology approaches to design phages from scratch.
Ethical considerations accompany these technological advances. The potential to engineer "designer microbiomes" raises questions about enhancement versus therapy. There are also concerns about phages transferring genes between bacteria in the gut, though current evidence suggests this occurs at very low rates with therapeutic phages.
Looking ahead, the field is moving toward personalized phage formulations tailored to individual patients' microbiome profiles and specific pathogens. Advanced sequencing technologies enable rapid identification of both harmful bacteria and their phage vulnerabilities, potentially turning around customized treatments in days rather than weeks.
The economic implications are equally transformative. While development costs are high, successful phage therapies could reduce healthcare expenditures associated with chronic infections, hospital stays, and antibiotic resistance management. Some analysts predict phage products may follow a similar trajectory to monoclonal antibodies - from niche treatments to mainstream therapeutics.
As research progresses, interdisciplinary collaboration becomes increasingly vital. Virologists, microbiologists, gastroenterologists, computational biologists, and regulatory experts must work together to translate laboratory successes into clinical reality. Several biotech startups and pharmaceutical giants are now investing heavily in phage-based solutions, signaling growing confidence in the approach.
Public perception presents both challenge and opportunity. While "virus therapy" might initially alarm some, educational initiatives can highlight phages' natural presence in our environment and foods. In fact, humans routinely consume billions of phages daily without adverse effects, as they naturally occur in water, vegetables, and fermented products.
The coming decade will likely determine whether phage therapy fulfills its promise as a precision weapon against gut pathogens. With antibiotic resistance rendering our current arsenal increasingly ineffective, these viral predators may offer a sustainable, evolution-approved solution to one of modern medicine's most pressing challenges.
From laboratory curiosity to clinical reality, bacteriophage therapy represents a paradigm shift in how we combat microbial threats. As research continues to unravel the intricate relationships between phages, bacteria, and human hosts, we may be witnessing the dawn of a new era in precision medicine - one where nature's smallest predators become our most sophisticated allies in maintaining gut health and fighting disease.
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025