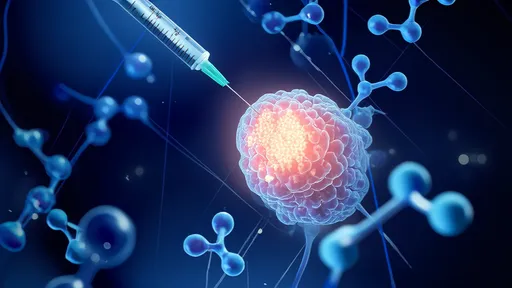
In a groundbreaking development that could redefine how we approach aging, scientists are making significant strides in the development of senescent cell vaccines – a novel immunotherapy designed to train the immune system to recognize and eliminate so-called "zombie cells." These dysfunctional cells, which accumulate with age, refuse to die while secreting harmful inflammatory compounds linked to nearly every age-related disease. The emerging field of senolytic vaccines represents a paradigm shift from treating individual age-related conditions to targeting a fundamental hallmark of aging itself.
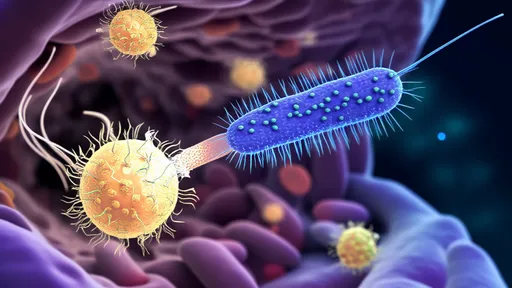
The biology of senescent cells reveals why they've become such a compelling therapeutic target. When cells experience irreparable DNA damage or other severe stressors, they typically enter apoptosis (programmed cell death). However, some cells enter an abnormal state of suspended animation – metabolically active but unable to divide. Like biological zombies, these senescent cells lurk in tissues, secreting a toxic mix of inflammatory cytokines, growth factors, and proteases known as the senescence-associated secretory phenotype (SASP). This molecular cocktail creates a pro-inflammatory microenvironment that damages nearby healthy cells and drives tissue dysfunction.
Researchers first recognized the potential of immune-mediated senescent cell clearance by observing natural processes in younger individuals. Our immune systems continuously survey tissues and eliminate senescent cells with remarkable efficiency during youth. However, immune surveillance declines with age, allowing zombie cells to accumulate. This insight led to two parallel therapeutic approaches: small-molecule senolytics that chemically induce senescent cell death, and the more sophisticated vaccine strategy that empowers the immune system to regain its natural surveillance capacity.
The vaccine development process begins with identifying senescent cell surface markers that distinguish them from healthy cells. Scientists have discovered several proteins that become uniquely expressed or modified on zombie cells, including uPAR, B2M, and specific glycoprotein patterns. These markers serve as the immunological "wanted posters" that vaccines present to the immune system. Advanced bioengineering techniques then create vaccine formulations that may combine these antigens with adjuvants or employ viral vectors to stimulate robust immune memory against senescent cells.
Recent preclinical studies demonstrate remarkable potential. In aged mouse models, senolytic vaccines reduced senescent cell burden by 50-70% across multiple organs while significantly lowering inflammatory markers. Treated animals showed improved physical function, including greater grip strength and endurance on treadmill tests. Perhaps most intriguingly, vaccinated mice exhibited delayed onset of age-related conditions – from cataracts to cardiovascular dysfunction – suggesting these vaccines might not just treat but potentially prevent multiple diseases simultaneously.
The translational challenges remain substantial. Unlike pathogens, senescent cells originate from the body's own tissues, raising concerns about potential autoimmune reactions. Researchers are addressing this through sophisticated antigen selection and immune tolerance mechanisms. Another hurdle involves ensuring the vaccine stimulates cytotoxic T cells and antibodies capable of penetrating the dense, fibrotic microenvironments where zombie cells often reside. Ongoing clinical trials are carefully monitoring these safety parameters while assessing optimal dosing regimens.
If successful, senescent cell vaccines could revolutionize geriatric medicine. Unlike traditional vaccines that prevent infectious diseases, these would represent a new category of therapeutic vaccines for age-related degeneration. Patients might receive periodic boosters throughout adulthood to maintain immune surveillance, potentially creating a cumulative benefit that slows multiple aspects of biological aging. The approach also offers advantages over chemical senolytics by providing more targeted action with potentially fewer side effects.
The broader implications extend beyond individual healthspan extension. By targeting a root cause of chronic inflammation ("inflammaging"), these vaccines might reduce societal healthcare burdens associated with multiple comorbidities in aging populations. Pharmaceutical companies are investing heavily in the space, with some analysts projecting the senotherapeutics market could exceed $50 billion annually if clinical efficacy is proven. Ethical discussions are emerging about equitable access and whether such interventions should be considered preventative healthcare rather than elective treatments.
As research progresses, scientists caution that senolytic vaccines won't be a panacea for immortality. Aging involves multiple interconnected biological processes, and eliminating senescent cells represents just one piece of the longevity puzzle. However, by restoring the immune system's natural ability to maintain tissue homeostasis, this innovative approach may help compress morbidity and extend healthspan – allowing people to remain vigorous and disease-free later into life than ever before.
The coming decade will prove critical as ongoing human trials evaluate safety and efficacy. With multiple biotech companies and academic centers racing to develop optimized formulations, the first senolytic vaccines could enter clinical practice within 5-7 years if current trajectories hold. This emerging frontier of immunogerontology exemplifies how understanding fundamental aging biology may yield transformative medical interventions that redefine what it means to grow older.
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025
By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025

By /Jul 18, 2025